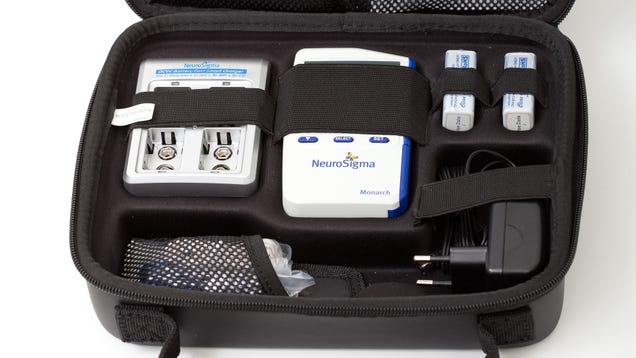
Last week, the Food and Drug Administration cleared the Monarch external Trigeminal Nerve Stimulation (eTNS) system, a medical device that provides mild brain stimulation to treatment attention deficit hyperactivity disorder in children ages 7 to 12.
from Gizmodo http://bit.ly/2Y7043h
EmoticonEmoticon